For Researchers
- Is IRB Review Required?
- FAQs
- Guidance
- Forms & Instructions
- Kuali IRB
- IRB Reliance | Single IRB Review
- EBP/QA/QI Projects
- Stem Cells and Human Fetal Tissue
Contact | IRBRely@ucsd.edu
In order to maintain regulatory compliance, and to help facilitate human research initiatives, the Reliance Team provides resources and support for researchers engaging in reliance activities with External collaborators. The resources and information found on this page provide a complete overview of the reliance process.
The goal is to enhance and streamline the IRB review process in the context of multi-site research so that research can proceed as effectively and expeditiously as possible. Eliminating duplicative IRB review is expected to reduce unnecessary administrative burdens and systemic inefficiencies without diminishing human subjects protections.
If you need additional information or have questions, please contact us at irbrely@ucsd.edu.
A review performed by one institution (IRB of Record) for multi-site research to establish the expectation that a single IRB (sIRB) of record will be used in the ethical/scientific review of non-exempt human subjects research protocols.
It does not substitute Investigator responsibilities or other institutional requirements. Investigators are still responsible for conducting research in compliance with regulations, laws, and institutional policies (local context) for the protection of human subjects in research. Should a project involve other review entities/committees such as OCTA, OCAA, PRMC, COI, etc. it is still the Investigator’s responsibility to ensure that these entities/committees have been informed of a required review, as appropriate.
A reliance agreement is a formal, written document that provides a mechanism for an institution engaged in research to delegate institutional review board (IRB) review to an independent IRB or an IRB of another institution. Authorization agreements may be for a specific study, a specific research, or for specific classes or categories of research. They describe the responsibilities of the relying institution and researcher as well as the responsibilities of the reviewing IRB and its institution.
NOTE: Reliance agreements are executed by an authorized Signatory Official/Institutional Official (IO) or designated IRB representative. Investigators cannot sign themselves.
The Streamlined, Multisite, Accelerated Resources for Trials IRB Reliance platform (SMART IRB), is designed to harmonize and streamline the IRB review process for multisite studies, while ensuring a high level of protection for research participants. SMART IRB is not an IRB, but a master reliance agreement with hundreds of participating institutions. The SMART IRB master reliance agreement replaces the need to have an individual Institutional Authorization Agreement (IAA).
Please review the list of Participating Institutions to see if your collaborator’s institution is a member of SMART IRB. Additional resources are available on the SMART IRB Learning Center webpage.
Acknowledging the use of the Smart IRB Agreement can be accomplished one of two ways:
The SMART IRB ORS allows research teams to submit an electronic request to execute reliance under the terms of the SMART IRB master reliance agreement. The request is typically initiated by the lead study team whose IRB will be serving as the IRB of Record for all sites. Each person requiring access to the online reliance system will need to submit a separate request for access to the system. If you've requested access and haven't received confirmation within a week please contact irbrely@ucsd.edu
Request Access: https://app.smartirb.org/smartirb-app/register
Sign In: https://app.smartirb.org/smartirb-app/login
Important Login and Registration Information - Effective March 17, 2025
The new SMART IRB reliance system uses Single Sign-On (SSO) to log in. If you have not registered for a SMART IRB user account yet, the easiest way to register for an account is to use the Research Organization option, type "University of California, San Diego" and register using your ucsd.edu email to allow for the SSO process to complete without issue.
SMART IRB reliance system users with accounts prior to March 17, 2025
If your account was registered using your health.ucsd.edu email, you will need to log into the new SMART IRB system using the login.gov option. If you do not have a login.gov option that is linked to your health.ucsd.edu email, you’ll need to create one to log in to SMART IRB and have access to historical information.
If your account was registered using your ucsd.edu email, you will need to log into the new SMART IRB system using the Research Organization option and continue the Single Sign-on (SSO) verification.
If you are a part of the lead study team, the next steps are required to proceed with the request in the online system:
The IRB Reliance Exchange (IREx) is a web-based platform, supported by the Duke/Vanderbilt Trial Innovation Center (TIC), to facilitate Single IRB documentation, communication, and the exchange of information.
IRBs, coordinating centers, and study teams use IREx for the following features:
For more information please reference the following IREx resources:
For single IRB studies where UCSD will be the reviewing IRB, after UCSD IRB has approved the protocol, the UCSD PI must submit an amendment for participating sites to be reviewed. For each participating site, the following documents should be include in the amendment application in the Participating Sites tab:
The following documents will require review the UCSD IRB and should be uploaded to the Supporting Information section of the project:
To allow the UCSD IRB to provide a thorough review, participating sites should complete a local context questionnaire. If a participating site PI has questions about their site policies and requirements, they should contact their IRB reliance contact for assistance.
UCSD Local Context Questionnaire
For studies where UCSD is relying on an External IRB, an administrative application to rely on an External IRB must be submitted via Kuali before any reliance agreements are signed.
The application will be reviewed by the reliance team. Please note that this review is not a duplicative review. The reliance team performs an administrative review of the application to ensure that UCSD local context will be considered as part of the reviewing IRB’s review of UCSD as a participating site and to ensure reliance agreements have been completed, as appropriate. After the application has been accepted by UCSD OIA as an External Reliance study, the research team should ensure that post approval responsibilities are followed to include submitting: Continuing Renewals, Amendments that meet our criteria for administrative review, and Reportable events where the reviewing IRB has made a determination of Unanticipated Problems/Adverse Events/Findings of Continuing or Serious non-compliance/Suspension.
Researchers should plan accordingly as there are two steps to UCSD OIA Administrative Acceptance for relying on an External IRB: 1. Clearance to submit to external IRB and 2. Administrative Acceptance
Step 1 – For Clearance Submission, upload:
▪ Master Protocol/Research Protocol
▪ Overall study approval from the reviewing institution/IRB
▪ Investigator's Brochure/Package Insert/Instructions for Use, as applicable to the research
▪ Recruitment material template(s) from sponsor or reviewing institution/IRB, as applicable (for non-commercial IRBs only)
▪ Informed consent template(s) from sponsor or reviewing institution/IRB (for commercial IRBs only)
▪ Informed consent template with UCSD required institutional language added and tracked (for non-commercial IRBs only)
▪ Reliance agreement provided by the reviewing IRB (for non-commercial IRBs only)
UCSD OIA will issue the Clearance Notification via the Revisions Required comment in Kuali. After the reviewing IRB has approved UCSD as participating site, the application should be resubmitted in Kuali for acceptance.
Step 2 – Resubmit for OIA Administrative Acceptance, upload these additional documents:
▪ IRB approved - UCSD site specific informed consent document(s)
▪ IRB approved - UCSD site specific recruitment material document(s), as applicable
▪ IRB approval letter for UCSD as a relying site from the reviewing institution/IRB
UCSD OIA will issue an acceptance letter once we review the reviewing IRB's approval documents and verified UCSD local context has been addressed. NOTE: UCSD OIA acceptance AND required Institutional approvals must be completed before research can commence.
When relying on an External IRB, if UCSD will be consenting subjects, the IRB approved template should be updated to include our UCSD required language and requirements as outlined in the UCSD Reliance Consent Minimums document.
When submitting for Clearance to a non-commercial IRB, the research team should include the IRB approved Informed consent template with UCSD required institutional language added via track changes.
When submitting an application to UCSD OIA for Clearance to rely on a commercial IRB (Advarra/WCG), the research team should include the IRB approved template or the sponsor template. UCSD has created workflows with Advarra and WCG where they can add UCSD required language for UCSD sites. The commercial IRBs should also provide sites with a pre-review of the consent forms and may require additional input regarding UCSD required language.
If the sponsor is requesting a UCSD site-specific consent form be negotiated before submitting to the commercial IRB, the research team will be responsible for obtaining the IRB approved template from the reviewing IRB or sponsor and updating the IRB approved template to include UCSD required language and requirements as outlined in the UCSD Reliance Consent Minimums document.
UCSD IRB is willing serve as the IRB of Record for external sites when federally mandated, required by sponsors, or on a case-by-case basis.
How it works:
1 | Pre-SubmissionUCSD is willing to rely on an External IRB when federally mandated, required by sponsors, or on a case-by-case basis.
How it works:
1 | Pre-SubmissionStep 1 | UCSD PI Contacts the UCSD Reliance Team for a single IRB (sIRB) consultation
Step 2 | UCSD PI Submits Project for Review and Identifies UCSD Serving as the IRB of Record in the Kuali Application
Step 3 | UCSD IRB Approves UCSD Serving as the IRB of Record
Step 4 | UCSD PI Forwards IRB Approved Study Documents and Reliance Documents to Site(s)
Step 5 | UCSD PI Submits an Amendment in Kuali to Upload Documents from Site(s) for Review
Step 6 | UCSD IRB Approves the Addition of Relying Site(s) and Issues Approval Letter
Step 7 | UCSD PI Forwards Site-Specific IRB Approval Documentation to Site(s)
Step 1 | UCSD PI Submits External IRB Review Registration via Kuali
Step 2 | UCSD OIA Administrative Screening of Kuali External IRB Registration (Clearance)
Step 3 | UCSD PI Provides Site-Specific Documents to the Reviewing Site
NOTE: UCSD PI should communicate with UCSD ancillary committees as applicable. The OIA does not hold a submission for ancillary reviews to be completed.
Step 4 | External IRB Performs Review and Issues Approval
Step 5 | UCSD PI Submits External IRB Approval Notice and Approved Documents via Kuali
Step 6 | UCSD OIA Issues Acceptance of Commercial IRB’s Review and Approval
Step 1 | UCSD PI Submits External IRB Review Registration via Kuali
Step 2 | UCSD OIA Administrative Screening of Kuali External IRB Registration (Clearance)
Step 3 | UCSD PI Submits Site Package to the Commercial IRB
NOTE: No need to wait for ancillary reviews to be completed before submitting to the Commercial IRB; these reviews can be conducted concurrently.
Step 4 | Commercial IRB Processing and Consent Customization Review by the PI/Study Team
Step 5 | UCSD PI/study team Reviews Prepared Consent Form(s) and Provides a Response directly to the Commercial IRB
Step 6 | UCSD PI Submits Commercial IRB Approval Notice and Approved Documents via Kuali
Step 7 | UCSD OIA Issues Acceptance of Commercial IRB’s Review and Approval
After the UCSD IRB has approved an external site to rely on the UCSD IRB for a specific study, the UCSD PI retains certain obligations with respect to the UCSD IRB and the relying sites. These include:
After the UCSD IRB has accepted to rely on an external IRB for review of a specific study, the UCSD PI retains certain obligations for submitting information within the Kuali IRB system. These are detailed in the IRB Handbook in the section "What are my obligations after IRB approval?" All of these obligations apply to PIs who are relying on an external IRB except #14 as the definitions of what is reportable and the timelines in which they must be reported vary among IRBs.
The important submissions that need to be made in Kuali IRB after the UCSD IRB has accepted to rely on an External IRB include:
Special Note About Studies with a Coverage Analysis
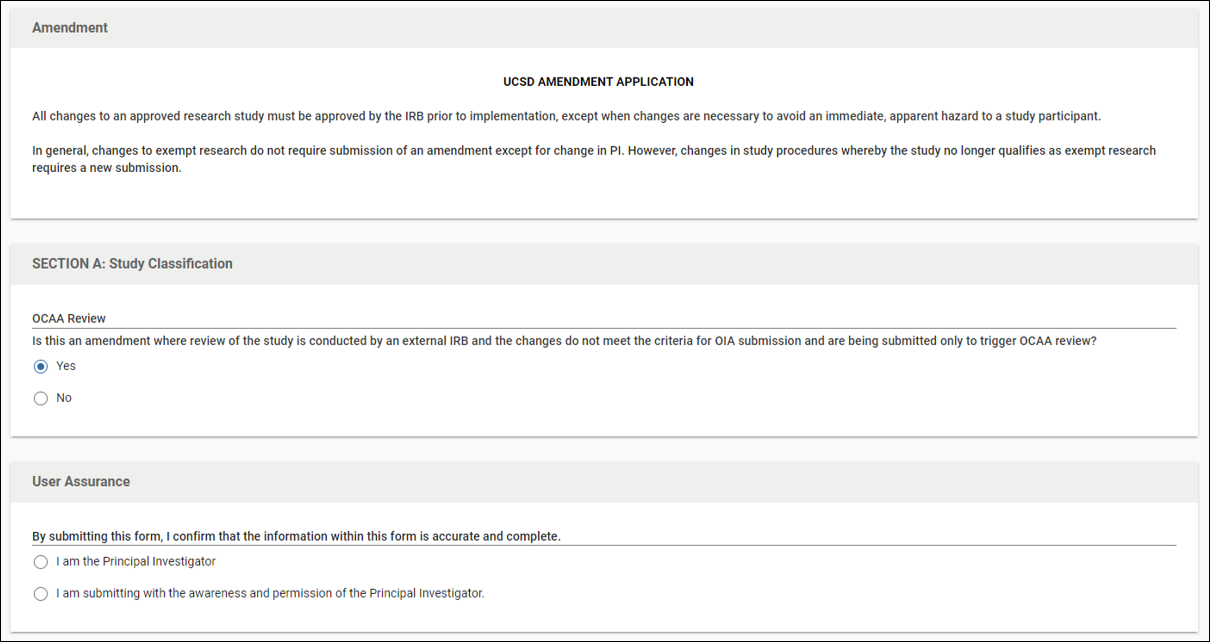
In addition to the above, the Office of Coverage Analysis Administration (OCAA) utilizes the Kuali IRB system to keep coverage analyses up to date for study teams. If your study requires a coverage analysis, please submit an amendment any time there is a change to the protocol, investigator's brochure (IB), or informed consent form (ICF).
To ease researcher and OIA staff burden in submitting and processing these amendments, OIA has a specific question in Section A of the Kuali IRB amendment application (shown below) that asks if this submission is only for OCAA review. If your study has a coverage analysis, is reviewed by an External IRB, and the amendment doesn't contain any of the items required to be reviewed by the UCSD IRB (listed above), answer this question as "yes." This will allow you to bypass all other amendment questions to save you time in submitting for OCAA review purposes.
If you have questions about whether OCAA review is required, please email OCAA directly at ocaa@ucsd.edu.
Multi-site (NIH funded research): means that the same research procedures (i.e., protocol) are being conducted at one or more domestic sites and that each site is under the control of a local participating investigator. This typically involves a lead site that receives the grant or contract directly from NIH and that then establishes a subaward or subcontract to each participating site. The research could be a clinical trial, an observational study, or a basic clinical research study.
Same Research Protocol: Protocols that address the same research questions, involve the same methodologies, and evaluate the same outcomes are considered to be the “same research protocol.” Additionally, sites that are accruing research participants for studies that are identical except for variations due to local context consideration would be considered to be conducting the “same research protocol.” If a study involves a separate site for study coordination or coordination of data and statistical analyses and the site is conducting the same protocol as the other participating sites, then all sites would be expected to rely on the designated single IRB.
Collaborative Study: Human research involving more than one institution and/or site participating in the same research protocol, where each site completes a portion or portions of procedures.
Cooperative Research: Human research covered by (45 CFR 46) involving more than one institution and/or site. When conducting cooperative research, each institution and/or site is responsible for safeguarding the rights and welfare of human participants and for complying with this provision. Any institution and/or site located in the United States that is engaged in cooperative research must rely upon approval by a single IRB for that portion of the research that is conducted in the United States.
IRB of Record: The IRB that is responsible for the ethical review of human research on behalf of an institution and/or site or individual investigator.
Lead Site: The primary awardee of a federally funded grant who is responsible for identifying the selected sIRB for cooperative research. For non-federally funded research this role is identified as the primary institution and/or site whom develops a research protocol.
Participating Site: An institution or site engaged in multi-site research, where a local investigator is responsible for the conduct of human research at their institution or site.
Relying IRB: An IRB that has designated through an agreement to cede review to an external IRB for a particular study