FAQs
This page is designed to bring together the most commonly posed questions which come from researchers to the Office of IRB Administration. OIA will periodically update this page as new topics emerge so the content may change over time based on feedback and evolving thoughts on the topics contained on this page.
Here at the OIA, we have put together a number of Knowledge Based Articles (KBAs) to help you with your most frequent questions. These are great resources to check out if you are having questions throughout the IRB submission process. These documents have step by step directions and photos to assist you with many of the tasks that need to be completed when making a submission to the IRB. Here is your one stop shop for all Kuali IRB KBAs: Kuali IRB - Research (ucsd.edu). Many of these KBAs will be linked in the answers provided below.
General IRB FAQs
Q: Do I need to submit my research for IRB review?
A: Here at the IRB, we review studies of human subjects research for which UCSD or RCHSD are engaged. All other research does not need to be reviewed by us, though there may be others at the institution that are required to review it.
Please refer to the two guides below to help you determine if your research is considered Human Subjects Research
Worksheet: OIA Worksheet 310
Diagram: Determining Whether a Proposed Activity is Human Research
If you went over the guides and you have decided that your research is human subjects research, the next step is to determine whether UCSD and/or RCHSD are engaged in the human subjects research. Please refer to the worksheet below to help you determine if UCSD and/or RCHSD is engaged in the human subjects research
Worksheet: OIA Worksheet 311
If your research is human subjects research and UCSD and/or RCHSD are engaged in the research, submission for review is required.
Still unsure if you need IRB review? Write to us at irb@ucsd.edu and we'll be happy to help.
Q: I've determined that my research doesn't need to be submitted to the IRB, but I need an official letter. What do I do?
A: If you've determined that your research isn't human subjects research, submit an application using the steps in the link below for an "Activity Not Human Subjects Research" determination.
If you've determined that your research is human subjects research but UCSD and/or RCHSD is not engaged in the human subjects research, submit an application using the steps in the link below for a "UCSD Not Engaged in Research" determination.
Q: Who can serve as Principal Investigator on research projects?
A: Generally, only UCSD faculty and staff members are eligible to serve as the Principal Investigator.
The current UCSD PPM 100-5 (https://adminrecords.ucsd.edu/ppm/docs/100-5.HTML) defines a "Principal Investigator (PI)" as "The person working on behalf of UC San Diego who is responsible for the ethical conduct of the research and for carrying out the responsibilities described in this policy. Generally, in order to serve in this capacity for human subject protections an individual must be eligible under UC San Diego policy to submit proposals for extramural support and serve as PI. The protocol PI (for human subject protection purposes) does not have to be the same person as the award PI (for funding purposes)."
Q: Can Graduate Students serve as Principal Investigator?
A: No, graduate students will need to identify an eligible UCSD faculty/staff member to serve as PI for their projects. PhD students may be listed as Co-Investigator under the Study Personnel section on the Kuali application. This applies to PhD students, graduate students, medical students, residents, and Post-Doc/Medical Fellows. Most graduate students list their mentor or advisor as PI.
NOTE: The only standing exception to this rule is that graduate students in the SDSU/UCSD JDP may be listed as the PI in the Kuali IRB application when SDSU will be the IRB of record, the UCSD IRB will rely on the SDSU IRB, and the student does not have a faculty mentor at UCSD.
Q: How do I know if my mentor/advisor is eligible to serve as my PI?
A: You can check if your PI is eligible at the following link: https://blink.ucsd.edu/research/finding-funding/pi-eligibility.html#Who-is-eligible-for-automatic-P
Q: I want to add Key Personnel to my approved project. When can the new Key Personnel participate in conduct of research activities?
A: New Key Personnel are eligible to participate in conduct of study procedures once ALL of the following are true:
- An amendment to add them to the Study Personnel section of the project’s Kuali IRB application has been submitted (you must hit Submit – unsubmitted amendments do not meet this requirement).
- The new Key Personnel have completed the required Human Subjects CITI training.
- They have received training from the PI/designee on the protocol and for their role.
- Any other documentation held and maintained by the study team is updated to reflect their involvement, e.g., the Delegation of Authority Log has been updated.
NOTE: The new Key Personnel will only have access to the Kuali IRB project record per your chosen settings (Read Only or Full Access) once the amendment has been reviewed and approved by OIA.
Q: What do I need to do to setup a Data Sharing or Material Transfer Agreement for my project?
A: If data/specimens will leave UCSD/RCHSD, an agreement must be in place first. Studies with a Clinical Trial Agreement or Grant award should have their study teams contact the appropriate office if they aren't sure whether the specific contract/award covers data/specimen sharing. Unfunded studies at UCSD will need to obtain a DUA/MTA from OCGA before data or specimens are shared outside of UCSD.
RCHSD researchers should reach out to research@rchsd.org to establish data/specimen sharing agreements.
Q: How can I request a copy of the eIRB approved documents we are required to submit with the one-time transfer amendment?
A: OIA has an eIRB document request form research teams can fill out located at the following link: https://support.ucsd.edu/its?id=sc_cat_item&sys_id=b28ff7411b349190d139113fad4bcbcd
Please Note: As of September 1, 2023, the Legacy eIRB system has been taken offline unexpectedly. ITS is working on a solution but there is not currently an estimated timeframe in which we expect the access to the Legagcy eIRB system to be restored. In the meantime, the files are secure and accounted for; however, access is significantly limited. OIA can assist researchers needing records for urgent issues (e.g. FDA inspections) and we kindly request that all others wait until ITS is able to restore OIA access to the Legacy eIRB system.
Q: Which trainings are researchers required to complete?
A: Human Subjects Research trainings to satisfy UCSD IRB training requirements are completed in the Collaborative Institutional Training Initiative (CITI) system. The UCSD IRB requires the completion of either the "Biomedical Research - Basic/Refresher course" or "Social & Behavioral Research - Basic/Refresher" course training, whichever is more applicable to the research activities of the project.
The other CITI courses are optional but may be found beneficial to those conducting research. Should you conduct research involving patient populations or conditions and drugs, devices, or agents, it is also strongly suggested that you complete the Good Clinical Practice Course that is extremely helpful in providing an excellent foundation for research and may be required by a study sponsor. The required CITI training OIA staff verifies completion on are only for the above mentioned Basic/Refresher courses. The Office of Compliance and Privacy may have additional required trainings, which can be reviewed here: https://pulse.ucsd.edu/departments/compliance/education-training/research-compliance-training/Pages/CITI.aspx
Q: I believe I've completed the required training but I received an Action Item that it isn't complete. Why does OIA think I still need to complete training?
A: OIA reviews the information provided to our office from the CITI website to verify the fulfillment of the required trainings. If you receive an Action Item about incomplete training but you believe you have completed the training in the last 3 years, the issue could be related to affiliating your CITI training account with our institution. Please be sure to double-check that you have affiliated your CITI account with University of California, San Diego. Please find directions here to affiliate your account with our institution: https://support.citiprogram.org/s/article/how-to-add-change-your-affiliated-institution-or-transfer-completions
Alternatively, it could be that some of the modules in the required training are incomplete or have since expired. Be sure to double-check your "Course Completion History" or "Modules Completed" on the CITI website to confirm if your required training is up-to-date. If you aren't sure what modules or courses are outstanding, please email irb@ucsd.edu for assistance in identifying the course/module name that is due for completion.
Q: Do the required CITI trainings cost a fee to complete?
A: No, OIA's required CITI training courses are free of charge for researchers that affiliate their CITI training account with our institution. If you are being asked to pay a fee, double-check that your CITI training account is properly affiliated with University of California, San Diego (https://support.citiprogram.org/s/article/how-to-add-change-your-affiliated-institution-or-transfer-completions).
If you are seeing options/prices after you complete a training module, please note the CE (continuing education) credits for purchase at the bottom of the screen can be ignored. Those credits are only for renewing licenses.
Q: I'm a graduate student I haven't fully figure out how to design my research project. Can I submit my study in Kuali while I'm still figuring out how to do the research?
A: The best resource for assistance in designing a study and developing a research plan/protocol is the eligible UCSD faculty/staff identified as the Principal Investigator for the project. Faculty/staff serving as PIs for graduate students' research projects can provide guidance from their research experience. OIA and the IRB are not set up to provide research design assistance.
General Kuali FAQs
Q: I'm having trouble navigating Kuali, can someone at OIA help me?
A: OIA has Kuali training videos available on our webpage.
There is a library of resource articles that help users with questions about the Kuali Access process, submitting applications for New studies, submitting Amendment requests and Renewal applications and more: https://support.ucsd.edu/research?id=kb_category&kb_category=6a80ba0a1b99f01048e9cae5604bcb52
For additional assistance with Kuali system issues, please contact irb@ucsd.edu.
Q: What is the difference between Kuali Research (KR) and Kuali Protocols (Kuali IRB)?
A: Please note that although Kuali Research (KR) and Kuali Protocols (also called "Kuali IRB") are related in some processes, they are managed by different UCSD offices. OIA manages Kuali Protocols, which houses the Kuali IRB application process. Kuali Research (KR) serves as the official institutional record of proposals, awards and subawards. KR integrates and aligns all elements of the research lifecycle including PI certification, detailed budgets, scope of work, Conflict of Interest, IRB, Export Controls, sponsor requirements and other compliance elements essential for complying with Federal, State and UC Regulations and Policies. For assistance with Kuali Research issues, please contact researchadmin@ucsd.edu and for assistance with Kuali Protocols, please contact irb@ucsd.edu.
Q: How can I search for an award in Kuali?
A: When responding to the questions in the Funding section of the Kuali application, the system will prompt you to utilize the Funding Gadget that links the award/grant to the Kuali Research Record. Please review the guidance provided in the following article on How to use the Funding Gadget.
Additionally, Kuali Research has articles for help with Searching for an Award in Kuali Research and How to determine if your Award is a Gift or a Grant.
If you still need assistance finding your award in the Kuali IRB Funding section, please contact irb@ucsd.edu for help. For assistance with finding an award in Kuali Research, please contact researchadmin@ucsd.edu for assistance.
Q: How do I find approved Consent Forms/documents with approval stamps in Kuali?
A: With the switch from the eIRB system to the Kuali IRB system, research teams are now able to retrieve their approved documents directly from the Kuali application at their convenience. Please proceed by logging in to Kuali IRB and selecting Protocols, then click on the study title link you'd like to view to open the application. Then scroll down to the Supporting Information section and click on the file name link you'd like to retrieve. The PDF formatted files will open in a pop-up window preview and there will be a download button at the bottom left corner of the pop out window.
The approval stamps are located in grey, as a watermark along the bottom of the document. Please note, the Kuali system is unable to stamp documents that are submitted in landscape format. Please submit documents in portrait format, no larger than 4.6 MB in size to allow the Kuali system to provide approval stamps. Additional guidance is available in the How to Locate Approved Document set in Kuali IRB article.
Q: I submitted an amendment and it was approved but now when I try to open up the approved documents, it continuously loads and never opens. Why can't I open this approved document?
A: Sometimes documents that get stamped in Kuali IRB (i.e. consent forms, assent forms, and recruitment materials) won't load properly after the IRB has approved their study because the files are too large. When a file is too big and Kuali tries to apply the approval stamp, Kuali can't load the final document. To avoid this happening, please keep all file sizes for documents to be stamped less than 4.6 MB.
If you are experiencing this issue, please submit an amendment with the form(s) that won’t open in a smaller file size so they can be stamped and able to be downloaded. If you urgently need a copy of the approved study documents with a visible approval stamp, please contact irb@ucsd.edu for assistance.
In the long term, the Kuali IT development team is aware of this issue and are currently working on a fix to prevent this file size capacity limit issue in the future.
Q: How do I create filters for my studies in Kuali?
A: Creating filters involves using the "Advanced Filter" feature in Kuali IRB. For detailed instructions on how to create filters in Kuali IRB, follow the link below:
We in OIA have also developed some common filters in Kuali IRB that may be helpful for most folks. To add these filters to your Kuali IRB account, follow the link below for directions:
Q: I have an old study in the Legacy eIRB system that I need to transfer to the new Kuali platform. How do I do that?
A: Here's how to transfer an existing study to Kuali from Legacy eIRB: https://support.ucsd.edu/research?id=kb_article_view&sys_kb_id=deb3161e1b73c910d1b0a935604bcbe7&sysparm_article=KB0033578
Q: I am not considered eligible to serve as a PI, but I am a Co-PI. How do I fill out the Kuali application?
A: Federal awards allow for Co-PIs and multiple PI plans; however, the UCSD IRB and the Kuali system do not support listing multiple PIs. A single Principal Investigator must be identified and Co-PIs may be listed as Co-Investigators under the Study Personnel section.
Q: Can I turn off Kuali email notifications?
A: The Kuali system is designed to distribute all study notifications to all study team members who are listed in the Study Personnel section of the IRB application. This is hard coded into the software by Kuali and not something that we at UCSD have any control over to turn off. Our recommendation is that folks who don't need to receive these email notifications should setup email inbox rules to have these notifications safely sent to a folder so that they don't clutter up their inbox.
Q: I'm not receiving Kuali notifications for the project(s) I'm involved in. Is there a way to change my contact settings so that I start getting these Kuali notifications?
A: There are a few items to check to help troubleshoot an issue of not receiving Kuali notifications. First, check you Kuali user account page to see if the email listed for your account matches your email address. To do this, navigate to https://ucsd.kuali.co and click on the "Users" tile. When the page refreshes, there will be a search bar towards the top of the page. Enter your name as [Last Name], [First Name] and click on your name to view your account page. Note that Kuali user accounts listed with "@ucsd.edu" instead of "@health.ucsd.edu" is standard for the @health vs @ucsd email parentage and notifications should still flow through to your @health inbox.
Next, check your Kuali user account information matches your Kuali account information listed in the Permissions tab at the top of a Kuali application you're not receiving notifications on. If the user account information doesn't match what is listed in the Permissions tab, please contact irb@ucsd.edu for assistance.
Additionally, please confirm if any Kuali notifications have been routed to your spam/junk folders or if you have any email rules setup in your inbox that may be filtering the notifications out of view.
Finally, confirm with the PI/research team members that you are listed as Study Personnel for the project(s) you aren't receiving notifications on. Only contacts included in the Study Personnel section of the IRB application will have Kuali notifications sent to their email.
As always, please contact irb@ucsd.edu if additional assistance is needed.
Q: My project was approved in the eIRB legacy system, but I don't see it when I log into Kuali. Do I need to fill out a new Kuali IRB application?
A: With the transition from the eIRB legacy system to the new Kuali IRB application, there was a migration process that occurred. OIA exported limited information from the eIRB legacy system to create shell records for studies in Kuali around May 2021. Although the export went smoothly, some users don't see all the studies they expect. Here's why (and what to do if it happens to you):
The first step for any user is to make sure no Advanced Filter or Saved Filters are limiting views while viewing your Manage Protocol dashboard in Kuali. The filter buttons to check are on the right of the Manage Protocols screen.
Next, if you are not the Principal Investigator (PI), the shell record will not be visible to you at first. This is because we could only export the PI information. If this happens to you, check with the PI. If the study is visible to them, they just need to quickly add you to the Permissions (see PI Permission Step by Step). Then, proceed with submitting the one-time transfer amendment to complete the transition to the Kuali IRB application (see How-To Submit the One-Time Transfer Amendment).
Finally, if you are the PI and a shell record is not visible to you, contact irb@ucsd.edu for help. It is likely you will need to submit a Kuali IRB application, as if it were a new study, however there is a box to check when starting the application under Facesheet Inclusion that reads, "I am transferring a study from the eIRB system" and OIA will assist with guidance on how to complete the Kuali application submission, as appropriate for your project's specific need.
Kuali Access
Q: I can't get into Kuali. Every time I try to sign in using my SSO, I am unable to. What's the problem?
A: Only UCSD staff and faculty are automatically given access to Kuali. If you are a student, volunteer, or a non-UCSD employee, you must request access to Kuali. Here is a link with general information on requesting access to Kuali:
Research Knowledge Base - Kuali IRB Access General Information (ucsd.edu)
Q: I'm a student at UCSD and I tried to login to Kuali, but was unable to. How do I get into Kuali?
A: Only UCSD staff and faculty are automatically given access to Kuali. As a UCSD student, you do not need access to Kuali unless you are making submissions to a study or for some reason need to view a study. If that is the case, please follow the link below for in-depth directions on how to request access to Kuali. Keep in mind, here at the OIA, we are unable to create, edit, or delete Kuali accounts.
Q: I am affiliated with UCSD, but I'm not UCSD staff. How do I request access to Kuali?
A: For those affiliated with UCSD, but not an employee of UCSD (i.e. volunteers, SIO, Scripps, RCHSD), please follow the link below for directions on how to request access: Research Knowledge Base - Kuali Access for Non-Employees (ucsd.edu)
NOTE: Collaborating researchers at other institutions and members of CROs or Sponsors cannot be provided with access to Kuali IRB. If a research team requires regulatory assistance beyond what is available with their department, OIA suggests reaching out to the ACTRI's Center for Clinical Research which can help with regulatory submissions. A request for services can be submitted here.
Q: What if I'm not sure who my DSA is?
A: UCSD has a great tool for looking up DSA's. Please visit the website below to be directed to the UCSD DSA search engine:
Q: I'm a DSA (Department Security Advisor) and someone who is neither UCSD staff/faculty or a student has requested access to Kuali. How do I go about adding an account to Kuali for them.
A: To start, there is a limited group of people that can request Kuali access outside of UCSD. These are the following individuals, outside of UCSD affiliated individuals, that can be sponsored for a Kuali account:
- RCHSD staff – They must go through a UCSD department to get sponsored. This is usually done through Pediatrics. Contact pedsdsa@health.ucsd.edu to request an account.
- Researchers at Scripps Medical Center who are looking to apply for SCRO review as Scripps has their own IRB. - The UCSD IRB will sponsor their access.
- Researchers from Scripps Institute of Oceanography – The UCSD IRB will sponsor their access.
- Students in the SDSU/UCSD joint doctoral program – The location in which their mentor is located is the IRB of record
As a DSA, if you need help giving Kuali access to non-UCSD researchers, please follow the link below for directions on how to do so: Research Knowledge Base - UCSD DSA Steps for non-UCSD Access Requests
Q: I am neither UCSD staff/faculty nor a UCSD student, but I need access to Kuali. How do I put in a request?
A: The UCSD IRB can only cover official UCSD volunteers, staff, faculty, or students or those at other institutions where we have specific policies that allow us to act on their behalf. Below is a list of individuals, outside of UCSD affiliated individuals, that can be sponsored for a Kuali account:
- RCHSD staff – They must go through a UCSD department to get sponsored. This is usually done through Pediatrics.
- Researchers at Scripps Medical Center who are looking to apply for SCRO review as Scripps has their own IRB. - The UCSD IRB will sponsor their access.
- Researchers from Scripps Institute of Oceanography – The UCSD IRB will sponsor their access.
- Students in the SDSU/UCSD joint doctoral program – The location in which their mentor is located is the IRB of record
All others will not be granted Kuali access.
For those who do fall into one of these categories, please follow the link below to request access to Kuali for non-UCSD users: https://support.ucsd.edu/research?id=kb_article_view&sys_kb_id=1b63ba941be5705048e9cae5604bcb89&sysparm_article=KB0033540
Q: I have a Kuali account, but I am experiencing odd functionality in Kuali, such as pressing the "Next" button when filling out a Kuali application and nothing happens. What should I do?
A: When a user does not have a Kuali account, but they login to Kuali using their SSO, sometimes a Kuali account is automatically created. However, the Kuali account has not been fully set up causing issues in the system. If you are experiencing these odd issues, please contact irb@ucsd.edu so we can assist you in getting your account fully set up.
Q: I made a change to the Principal Investigator assignment under the Study Personnel section and now I can't access my application anymore. What happened?
A: The Kuali system auto-updates permissions to Read-Only when a Researcher Role is selected for any role other than Principal Investigator. For example, if you changed your Researcher Role from PI to Co-Investigator, Kuali will auto-update your selected permissions to Read-Only access when that researcher role is selected. Please be sure to scroll down on the Study Personnel editor pop-up window and double-check the Full Access permissions check box is selected prior to clicking Done when making this edit. If you lose access permissions, please contact IRB@ucsd.edu for assistance. If your Kuali application is in a status that is open for editing, in some instances OIA team members can revert your permissions back to Full Access on your behalf to restore to your access to your application.
Kuali Permissions FAQs
Q: What are the Permissions Access settings and how do they work?
A: There are two types of Permissions Access options for Kuali IRB applications: Full Access and Read-Only.
Full Access permissions allow the user to make submissions like amendments/renewals/close requests and make edits to the application. Read-Only permissions limit the user to have view access to the application and they are not able to make submissions or edits to the application. Users can check their Permissions Access selections by viewing the Permissions tab at the top of the Kuali application, or by clicking the eye icon to the left of their name under the Study Personnel section of the application. If you see that both permissions are selected, the Kuali system will default to Read-Only access. Additionally, if the permissions selected in the Permission tab are mismatched with the permissions selected under the Study Personnel section, you will experience odd functionality in the Kuali application such as being able to submit an amendment but not able to reply to an Action Item.
If neither Full Access or Read Only access are selected, then the research team member will not be able to view or edit the Kuali application.
Q: How do I add or change someone's permissions to view or edit my study?
A: Here are step by step directions for how manage permissions in a Kuali protocol:
Submission FAQs
Q: I need an approval letter for my recently approved submission. How do I get one?
A: The great thing about Kuali is that when your submission has been approved in Kuali, be it your initial submission, your renewal, or an amendment, Kuali automatically generates an approval letter. To access that approval letter, please follow the directions below:
Q: My study got sent back with Action Items. How do I respond?
A: It is common for studies to be returned with revisions being requested by the reviewer before they can give an approval. An action item might be as simple as ensuring that you answered all parts of the Kuali application thoroughly or as complex as requesting that you revise part of your protocol to minimize risk to research participants. You can tell there are action items needing review when you look at your protocol in Kuali and notice orange/yellow circles in your Kuali application. If you press on the yellow/orange circle, the action item(s) will be visible on the right side of your screen. If you need to respond to the action item, please follow the link below for directions on how to do so:
If you see there are Action Items but do not have the ability to respond, it is likely due to the permissions settings selected for your account on the study. Please contact irb@ucsd.edu for assistance.
Q: How do I make a submission to the IRB?
A: All IRB submissions use Kuali platform. When submitting a protocol application to us, Kuali changes the protocol application depending on how you answer questions in Kuali so it is important to answer all questions correctly as it may populate another section.
Here are some resources to help you figure out how to start a new Kuali application and how to fill it out properly based on your study criteria.
How do you choose the right application?: https://support.ucsd.edu/research?id=kb_article_view&sys_kb_id=91c4be161b19f41048e9cae5604bcbaa&sysparm_article=KB0033558
Here are steps on how to fill out a new application: https://support.ucsd.edu/research?id=kb_article_view&sys_kb_id=a738986387c2a5d4825ac807cebb3515&sysparm_article=KB0033579
Here are steps on how to submit an application for Administrative Determination: https://support.ucsd.edu/research?id=kb_article_view&sys_kb_id=3bc6751d1b4b99d0d1b0a935604bcb32&sysparm_article=KB0034063
Q: How do I submit an amendment in Kuali?
A: The great news in that you no longer need to fill out any facesheets or cover pages to make a submission in Kuali. It's easy to submit an amendment directly through Kuali by following these step by step directions:
Q: I'm trying to submit an amendment but the section I want to amend is locked/unavailable. What do I do?
A: The way the amendment applications function in Kuali is to allow the submitter to select and isolate the specific application sections they want to edit in the amendment submission. Once the application sections are selected and the amendment has been submitted, those selected sections are locked for further editing until the amendment has been reviewed/approved, or if the submission is returned for revisions. This locking feature is intended to give users the ability to submit multiple amendments at the same time.
For example, your project has study personnel updates and wants to make a change in the study procedures that requires edits to the protocol document. Since the study personnel changes would be a minor amendment and take less time for review/approval, you can submit an amendment that has only the Study Personnel section selected and requests those study personnel updates. Then, you can submit another amendment that has only the Supporting Information section selected to submit the tracked changes version of the protocol and the clean version of the protocol for review/approval.
This functionality is intended to give research teams flexibility/more options in the amendment process, but when there are multiple amendments submitted at once, especially if one or more have been sent back requesting revisions, this section isolation/locking can get a bit confusing to manage. If there is a section that is locked that you need to edit, return to your protocol dashboard and search by the 6 digit Kuali project # to view the active versions of the project that are open. Click on each amendment version to identify the version that has the section you want open for editing. If you need assistance identifying if you should abandon/withdraw an amendment to unlock a section for editing, please contact irb@ucsd.edu.
Q: How do I submit a continuing review or renewal?
A: The terms "continuing review" and "renewal" are used interchangeably by OIA to refer to the application required to be submitted by investigators prior to expiration of a study to allow its continuance and the subsequent review by the OIA or IRB. The Kuali system has fields for two dates, an "expiration date" and a "continuing review date," for every study.
If your study has an expiration date, then continuing review or renewal is required. If your study is not reviewed and renewed before the expiration date, your study will expire and all research activities must halt until the study is renewed. Ensure you submit renewals at least 30 days before expiration to give the IRB enough time to review your study submission and send it back for any necessary revisions. The link below provides step by step directions on how to submit a renewal in the Kuali system.
If your study has a continuing review date, this means the Kuali system will send you a yearly check-in to ensure the Kuali record is up to date and the study is still active. Your study won't expire come the continuing review date, however it is the responsibility of the research team to make sure everything for their study is up to date. This includes all research staff's CITI training being up to date, ensuring any reportable events have been submitted to the IRB, and making sure your documents have up to date stamps among other things. If something is not up to date, then when you receive the annual Kuali notice, please submit an amendment to the IRB to make any of these updates. Additionally, if the study has closed, it is the responsibility of the research team to submit a closure application to OIA.
Note for studies relying on an external IRB: Studies where the UCSD IRB has ceded its review to an external IRB will not have an expiration date but will have a continuing review date in the Kuali system. Nevertheless, the study may have an expiration date or continuing review date in the external IRB's system. Researchers should ensure they're compliant with the external IRB's policies and procedures to keep their study current. When a study is renewed with an external IRB and the renewal approval is received, the approval must be submitted in the Kuali system as a renewal application to keep our records current.
Q: I'm submitting a renewal and I can't upload new documents or make study personnel updates. How do I submit this updated information?
A: With the switch from the eIRB legacy system to the new Kuali application, uploading study documents is no longer required in the renewal/continuing review process. If there are updates or modifications you would like to submit, please proceed with submitting an Amendment. Renewal applications do not allow edits to the Kuali application sections, including study personnel updates. The process for making changes to a project is designated to the Amendment request process. The Renewal application process does not allow for changes to be requested within the review, as it is only a process for updating the approval of the research. If you have additional questions/concerns, please contact irb@ucsd.edu.
Q: How can I be sure I've submitted my application in Kuali?
A: The best way to confirm if you've properly submitted an application in Kuali is to check the Status of the application. The status of a submission is shown in two places in Kuali: the Manage Protocols page in the Status column and at the top of the application when viewing a submission (See images below)
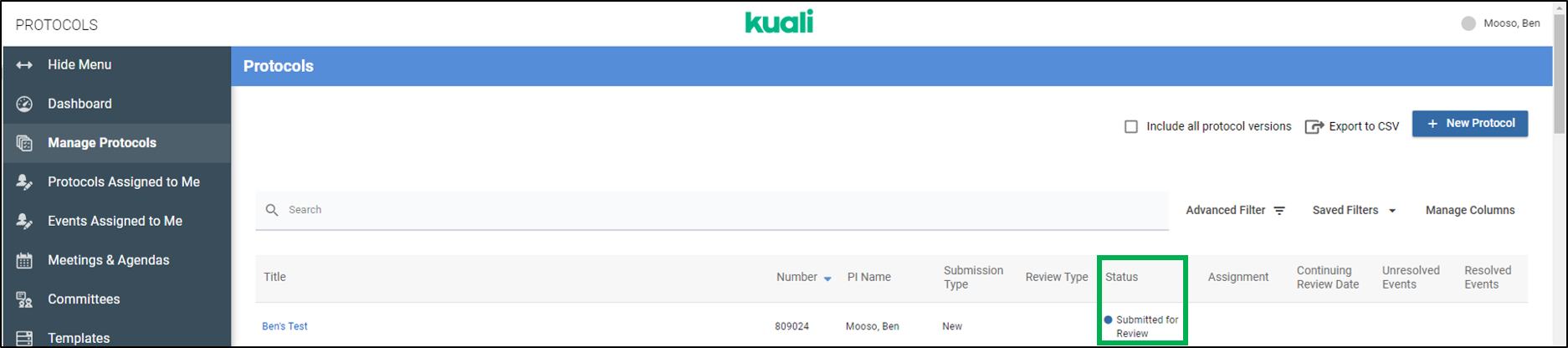
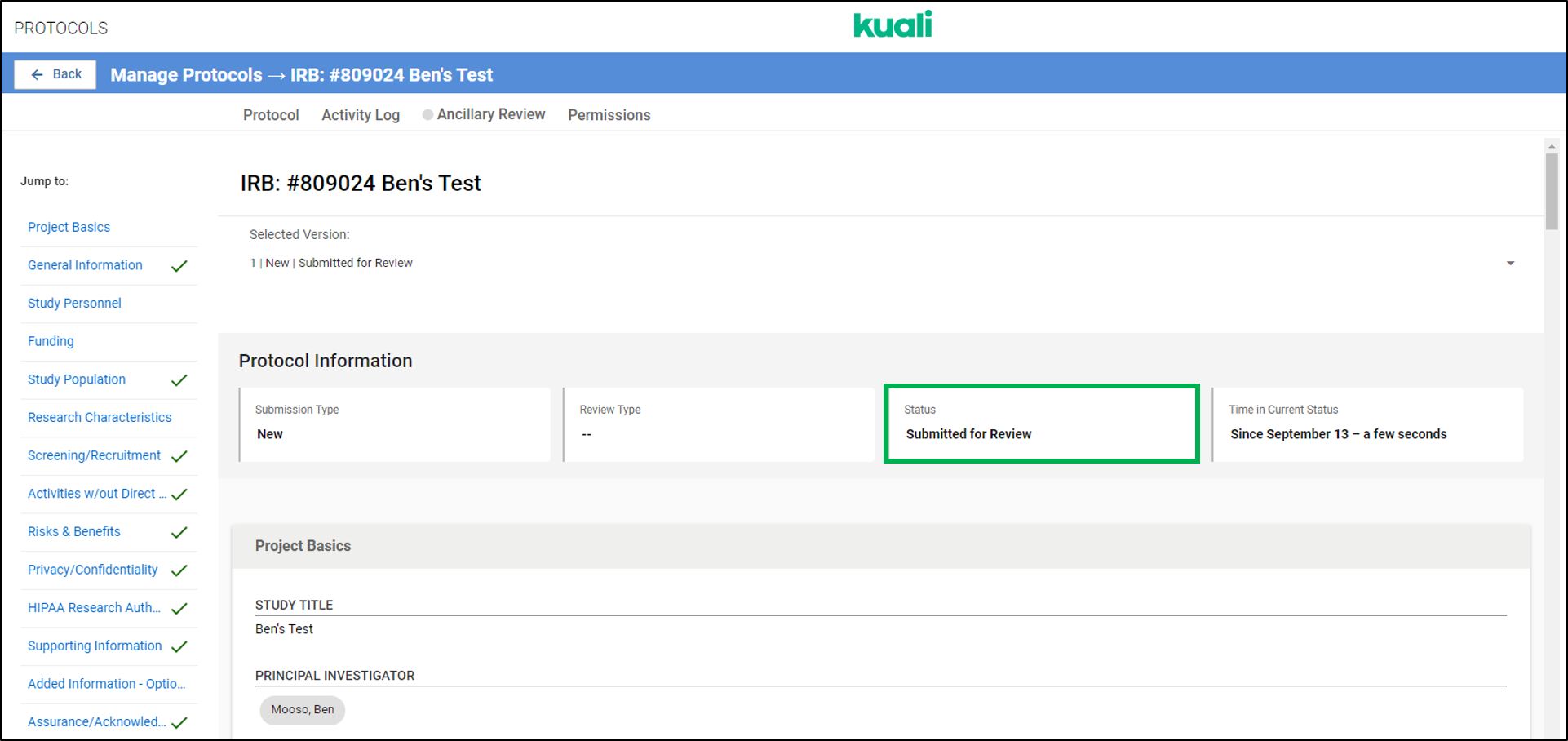
Statuses that confirm your application has been submitted include:
Submitted for Review
Resubmitted
Statuses that indicate you have yet to submit your application or it is currently open for editing/not submitted to OIA for review include:
In Progress
In Progress (Withdrawn)
Revisions In Progress
Returned to Researcher
Abandoned*
*studies with this status cannot be restored/recovered. Once a project is abandoned, it cannot be reopened.
Statuses that indicate your submission has been approved:
Approved
External Reliance
Exempt
Not Human Subjects Research
Colored dot key for Kuali Statuses on the Manage Protocols page:
Green = Approved
Blue = Awaiting review by IRB
Yellow = Researcher preparing to submit to IRB
Orange/Brown = Submission has been reviewed and IRB has asked the researcher to make some revisions
Grey = The study is closed OR a submission that has been abandoned OR a submission that was previously approved and has been superseded by a submission more recently approved
Q: Are there required forms or templates I need to use when submitting my Kuali application for review?
A: Starting Sept. 1, 2022, all new studies (except for secondary use studies which only require the Kuali application) are required to use the master protocol if one exists (e.g. from an industry sponsor, cooperative group, etc.). Otherwise, you must use one of the three new protocol templates that are available on OIA's forms page. Additionally, all new UCSD/RCHSD studies requiring an informed consent or assent document will be required to use the new ICF and assent templates, also available on the OIA website at the link provided.
Q: How do I know which Protocol template to use?
A: If the research activities in the study involve having the subject participate in an intervention as defined in OIA SOP OO1, use: Biomedical Interventional Protocol for clinical trials research (based on NIH template)
- NOTE: The only exception to this requirement is that studies involving a venipuncture as their only intervention may use the non-interventional template.
If the research activities are Biomedical in nature, but do not involve the subject participating in an intervention, use: Biomedical Non-Interventional Protocol for clinical research
If the research activities are primarily Social/Behavioral, for example interviews or survey research, use: Social/Behavioral/Educational Research (SBER) Protocoll
If conducting a secondary data analysis study, like a retrospective review of medical charts, that do not include any additional biomedical activities, a protocol template is not required for submission and all required information will be collected within the Kuali application.
Q: How do I know which Consent/Assent template to use?
A: If consenting participants is part of your research activities, use one of the following consent form templates to create your ICF. These templates are located under the UCSD Templates heading on our website:
Adult/Parent Permission Consent Form template - Use this form for participants aged 18+ or when parents will consent for a minor.
Adolescent Assent template - Use this form for participants aged 13-17 years old.
Child Assent template - Use this form for participants aged 7-12 years old.
Exempt Information Sheet template - Use this form for research that may qualify for an exempt determination. For example interview/surveys/educational research/benign behavioral interventions with adult subjects may use this consent form template.
Expanded Access Consent Form template - Use this form for expanded access treatment applications. For example, expanded access, managed access, treatment access, compassionate use, use this form.
Q: What assent form do I use for child under age 7?
A: For Children under the age of 7, we do not provide an assent template as children under 7 who are capable of indicating their preference most commonly do not have the reading comprehension for an assent document. Additionally, they are likely to not be able to sign their name, therefore we have not provided an assent template for children under 7 and this is the standard practice in this country. The study team may still need to write a note in their own regulatory documentation about the child’s capability, that the assenting process took place, and the child’s decision. Children in this age group may not be capable of assent due to their age.
Q: Do I have to use the UCSD or UCSD/RCHSD protocol/consent/assent templates for my project?
A: The following are examples of times OIA will accept a non-UCSD protocol template:
-The protocol is provided by a study sponsor or a cooperative group, or
-The study has an IND or IDE from the FDA
-The project will be relying on another IRB for approval
-Exempt research projects that ask OIA to concur with another IRB's determination
OIA provided consent templates must be used when the UCSD or RCHSD teams will consent subjects, unless relying on an external IRB.
Q: How do I get approval for a Consent/Assent form in a language other than English?
A: We suggest you submit your consent/assent forms in other languages AFTER you receive initial approval on your English language version of your consent/assent form to avoid the financial and administrative burden of additional translations if/when revisions are required.
Be sure to include a language translation certification with any additional language documents. The certification of translation can be from the translation agency/company that provided the translation, or a letter from the research team member that translated the document(s) describing their language experience and fluency background. This certification document should include the signature of the translator along with the title, version, and date of each document translated.
Q: How do I get my Consent/Assent form translated into another language?
A: UCSD does not have internal translation services available to request for the translation of study documents at this time. Many research teams use translation agencies or companies for their document translation needs. It is also common for research teams to have a member of their study personnel that is fluent in the desired language to translate the required documents. Additionally, if the study is sponsored by industry, they may have translation services available for sites. OIA requires a copy of the translated document and a certificate of translation certifying the translation was conducted by a qualified individual. If you use a translation agency/company, they will typically provide a certificate of translation with their services. If you use a member of the research team or someone associated with the project that is fluent in the desired language, a letter from the translator describing their language experience would also be an acceptable form a certification of translation. This certification document should include the signature of the translator and the name of each document translated.
For information on the use for Short Form consent for non-English language speakers, please visit our website to find guidance on the short form consenting process on the following page under the Short Form Consent heading.
Q: I have completed the Kuali IRB application, but I'm not sure of some answers. Can OIA review my responses before I submit?
A: If the Kuali IRB application sections are completed and a user would like responses reviewed, please proceed with submitting the application in Kuali. It's best to get into the specifics of what and how exactly to revise/change with a reviewing analyst, which is not assigned until the project is submitted in Kuali IRB. Please note that it is to be expected to be asked for revisions/changes during the review process.
Q: I can't see an option to Reply to Action Items in Kuali, why can't I submit responses?
A: Users may be asked to submit responses to Action Items in Kuali, but not see an option to Reply or see how they can follow the guidance provided in the article How to Respond to Action Items in Kuali IRB. This issue can be due to the user having Read-Only permissions selected, both Full Access and Read-Only permissions selected, or a mismatch of Full Access permissions selected in the Permissions tab and Read-Only selected under the Study Personnel section or vice versa, in the Kuali application. Please contact irb@ucsd.edu for assistance resetting your permissions selections if your application is in a status that is open for editing.
Q: I accidentally submitted/resubmitted my Kuali application, but I have more edits/updates to submit. How can I open my application back up for editing?
A: View the upper right side menu of your Protocol application for a button that says "Withdraw". If this button is available, click it to retrieve the application back for editing. If this button is not available (greyed out or not visible), please contact irb@ucsd.edu for assistance. OIA analysts have the ability to "Return to Researcher" when the Withdraw feature isn't available.
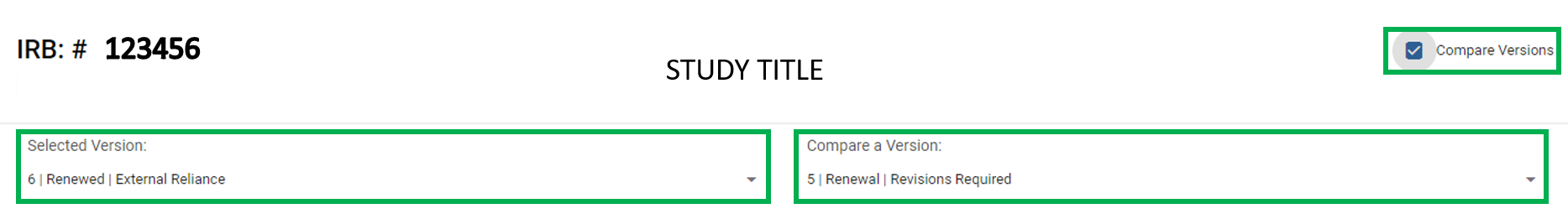
Q: How can I see what answers or documents have been changed on my Kuali application from previous submissions/approvals?
A: Kuali has a helpful feature that allows users to Compare Versions of the protocol in the same page view. Use the "Selected Version:" drop down located at the top of the Kuali application under the study title displayed in black text. Click on the text below Selected Version to choose the desired version/submission of the application. The "Compare Versions" feature is located to the right of the study title in black text, click the check box to populate the second version drop down feature. Click the text under the "Compare a Version:" to select the version/submission you would like to compare responses with. Once versions are selected under each drop down, the Kuali application will then show the differences between the two selected versions/submissions in red and green text. Kuali will show which responses are associated with the specific version with a colored icon to the right of the text noting the version, for example if comparing versions 1 and 2 of your application, Kuali will show "v1" or "v2" to indicate which responses/documents belong to each version/submission. For a visual representation of the drop down menus and compare versions checkbox, see screenshot below.
If you need assistance navigating the Kuali Selected Version or Compare Versions feature, or are having trouble deciphering how to proceed when multiple amendments are In Progress and Kuali application sections being locked for editing, please contact irb@ucsd.edu for assistance.
Q: My study is reviewed by an external IRB and we received an amendment that requires a change to the coverage analysis, budget, or clinical trial agreement. How can I submit a request for OCAA and/or OCTA Ancillary Review(s)?
A: To request an OCAA review only, proceed by making an amendment submission and respond "Yes" to the first question noting "Is this an amendment where review of the study is conducted by an external IRB and the changes do not meet the criteria for OIA submission and are being submitted only to trigger OCAA review?" This question is commonly selected erroneously by researchers and OIA will Return for Revisions requesting confirmation that the purpose of the amendment is to trigger the OCAA review. Researchers can also add the "Added Information" section to their amendment and note the purpose of the amendment is to trigger OCAA review to avoid the Return for Revisions confirmation request.
If you have additional changes to submit AND need to request OCAA and/or OCTA Ancillary Review(s), respond "No" to the first OCAA question and respond with one of the below options to the question, “Will this amendment result in changes to procedures that are billed to the research participants or changes to the budget negotiated with the sponsor?”:
"Will result in changes to procedures billed to sponsor" = OCAA Ancillary review request
"Will result in changes to negotiated budget = OCTA Ancillary review request
"Both changes to procedures billed to sponsor and changes to negotiated budget" = OCAA and OCTA Ancillary review requests
Q: My study is reviewed by UCSD's IRB, how can I submit a request for OCAA and/or OCTA Ancillary Review(s)?
A: If the review of the study is conducted by UCSD’s IRB and you need to trigger OCAA or OCTA review, but have no other proposed changes to the study, please email irb@ucsd.edu to request OIA to add the OCAA/OCTA Ancillary Review to your protocol.
To request OCAA and/or OCTA Ancillary Review(s) along with other proposed changes to the study, proceed by making an amendment submission and respond "No" to the first question about triggering and OCAA review (this question is specifically for studies reviewed by an external IRB). Then, respond with one of the below options to the question, “Will this amendment result in changes to procedures that are billed to the research participants or changes to the budget negotiated with the sponsor?”:
"Will result in changes to procedures billed to sponsor" = OCAA Ancillary review request
"Will result in changes to negotiated budget = OCTA Ancillary review request
"Both changes to procedures billed to sponsor and changes to negotiated budget" = OCAA and OCTA Ancillary review requests
Q: I received a request for revisions to include a tracked changes version of a document. How do I create a tracked changes document?
A: Microsoft has a helpful guide on their tracked changes review features available on their website here: https://support.microsoft.com/en-us/office/track-changes-in-word-197ba630-0f5f-4a8e-9a77-3712475e806a Although Microsoft Word is the most common word processing application used, if you're using a different application to edit your documents, we suggest you visit the application's website for assistance in locating the tracked changes review controls available to create your tracked changes document.
Study Personnel FAQs
Q: Who should I include in my Study Personnel list in my Kuali application?
A: Kuali Application Submission Types have different requirements for who should be included on your protocol application.
For the following Kuali Application Submission Types, list the names of all UCSD/RCHSD employees conducting the research or acting as a point of contact on the study in the Study Personnel section:
- Human Subjects Research – Expedited/Full Board
- Request to Rely on non-UCSD IRB
- Expanded Access – Use of a Test Article for Treatment
- Stem Cell Research Oversight (SCRO)
For the following Kuali Application Submission Types, add the PI to the Study Personnel section. Do not list any other personnel in the Study Personnel section. If needed, add an administrative contact in Permissions:
- Human Subjects Research - Exempt Registration
- Activity Not Human Subjects Research
- UCSD Not Engaged in Research
- Indefinite Plans / Delayed Onset (IPDO) formerly known as JIT
- Emergency Use of a Test Article for Treatment
- Humanitarian Use Device for Treatment
Q: Should I include co-investigators/collaborators from other institutions/organizations?
A: Non-UCSD/RCHSD study personnel should not be added to the Kuali application since our IRB can only cover the research activities of official UCSD volunteers, staff, faculty, or students or those at other institutions where we have specific policies that allow us to act on their behalf. If these non-UCSD investigators need to participate in the research, they should either obtain IRB approval from their own institution(s) for their participation or will need to have their own institution(s) and the UCSD IRB enter into a reliance agreement. For help getting started with a reliance agreement, contact IRBRely@ucsd.edu.
When there is a collaboration with an outside investigator or institution, while the UCSD IRB cannot cover their research activities without a reliance agreement, the activities of the outside investigator or institution should be described in the Protocol or Kuali application so that the IRB can understand the totality of what will be done in the study and by whom.
Q: I am trying to add someone to the Study Personnel section, but their name/email is not populating in the search. What should I do?
A: The primary method for adding study personnel to the Kuali application is by adding them under the Study Personnel section of the application. When a contact does not populate in the dropdown search, it is likely because that person does not have an active Kuali user account. For UCSD students and other UCSD affiliated contacts that do not have a Kuali user account, the alternative method of adding them to the Kuali application is to add them to the Unnamed Person question prompt in the Study Personnel section, if that question is populated on your application.
If the Unnamed Persons prompt is not included, the alternative section to add them to is the Added Information section. Be sure to include the UCSD/RCHSD member's name, email, role/responsibility in the research activities. These alternative methods are available to avoid the administrative burden of having students create a Kuali account to be added to the Study Personnel section of the application, if they do not actually have a need to review/edit the protocol application in the Kuali system.
If the contact is in need of view/edit access to the protocol application, please see the how-to guidance on requesting a Kuali user account be created: https://support.ucsd.edu/research?id=kb_article_view&sys_kb_id=e494eb9f1b31bcd0506f64e8624bcb7e&sysparm_article=KB0033574
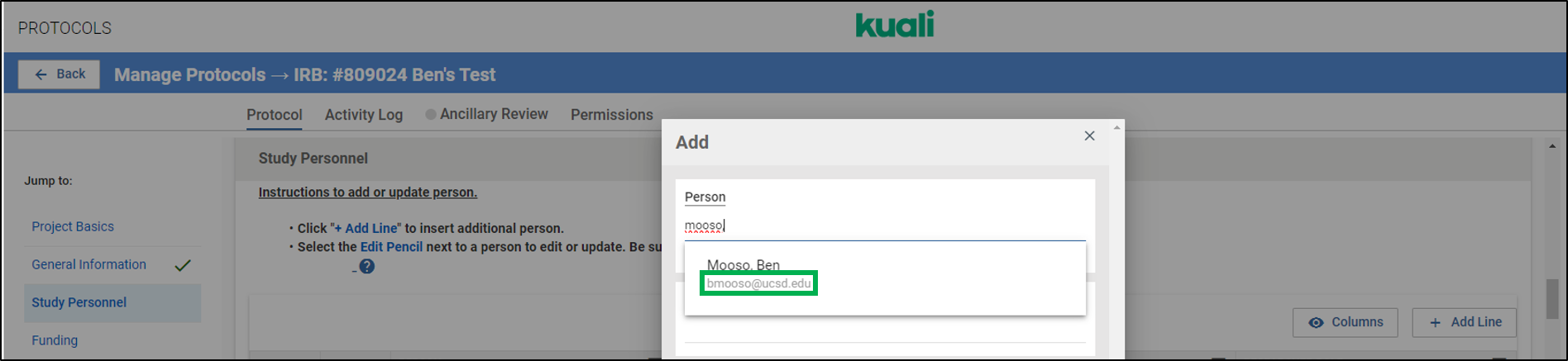
Q: I'm attempting to add someone to the Study Personnel section and multiple people populate in the search. How do I know which option to choose?
A: You can find the correct person from the options shown by making sure you select the person with the correct email address shown below their name. Please note, Kuali will only display the user's @ucsd.edu email address, even if they are a Health or Health Sciences employee.
Review Process FAQs
OIA encourages researchers to plan ahead and submit your Kuali applications early whenever possible. Please review the below Q&A for reference on how our review process functions and the estimated timelines for review/approval.
Q: I submitted my project in Kuali, when can I expect to hear back?
A: All Kuali submissions go through a screening and intake process prior to being assigned to an OIA analyst for review. The screening and intake process occurs within the first 1-3 days of submission during which potential errors and completeness are scanned for. Projects may receive requests for revisions before they can be assigned to an analyst for review. Once projects pass through the screening and intake process, they are assigned to a queue for review by one of our in house reviewers. Projects in queue are reviewed on a first come, first served basis. Outside of rush requests due to subject safety or funding stipulations, OIA is not able to accommodate requests for priority reviews due to the large volume of submissions our team processes on a regular basis.
Outside of the screening and intake process, we have approximate review timeline for the various types of Kuali application submissions. If you do not receive feedback within the expected review timelines below, please feel free to contact IRB@ucsd.edu to request a status update.
New Expedited/Full Board submissions are reviewed within 4-8 weeks from time of submission to our office
Initial/new External Reliance applications are reviewed within 5-10 business days from time of submission OR resubmission to our office
Minor administrative amendments (e.g. translations, study personnel changes, etc.) may be processed within 1-2 weeks from time of submission to our office
Amendments beyond minor administrative changes can take approximately 4-6 weeks from the time of submission to our office. If the amendment needs to be reviewed in an IRB Full Committee meeting, the review process can vary from 4-8 weeks from time of submission to our office.
Renewal applications should be submitted within 60-30 days prior to the study's expiration date to allow adequate time for review/processing
Q: Can OIA review a draft of my protocol and/or consent form documents before I submit my Kuali application?
A: We appreciate the effort to submit accurate and complete Kuali IRB applications. Please note that it is to be expected to be asked for revisions/changes during the review process. OIA is able to provide advisory assistance prior to Kuali application submission, however we do not edit applications or do a true review prior to Kuali submission during advisory reviews. Regulatory review of protocol and/or consent form documents is an activity included in the formal IRB review process itself.